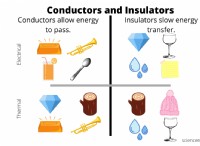
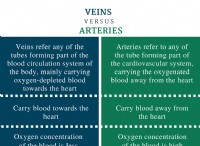
이유는 다음과 같습니다.
* 알칼리 금속 are in Group 1 of the periodic table (Li, Na, K, Rb, Cs, Fr).
* 그들은 하나의 원자가 전자를 가지고 있습니다 which they readily lose to achieve a stable electron configuration.
* Reaction with water: This single valence electron is easily donated to a hydrogen atom in water, forming a hydroxide ion (OH-) and releasing hydrogen gas. This reaction is highly exothermic, often causing the metal to ignite.
* Reaction with halogens: Alkali metals react explosively with halogens (F, Cl, Br, I) to form ionic salts. The single valence electron is transferred to the halogen atom, forming a stable ionic bond.
예 :
Sodium (Na) reacts violently with water to form sodium hydroxide (NaOH) and hydrogen gas:
2Na(s) + 2H₂O(l) → 2NaOH(aq) + H₂(g)
Sodium also reacts explosively with chlorine (Cl) to form sodium chloride (NaCl):
2Na(s) + Cl₂(g) → 2NaCl(s)